Breast cancer treatment approved for NHS use
 PA Media
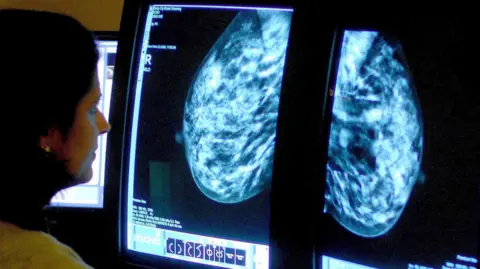
PA MediaSix new drugs, including a treatment for patients with advanced breast cancer, have been approved for use by the NHS in Scotland.
The Scottish Medicines Consortium (SMC) has given the green light to three new cancer treatments as well as a drug to treat a rare type of epilepsy. Medicine that can help prevent HIV infections has also approved.
This means the treatments can prescribed by NHS doctors across the country.
However, a drug which has been shown to slow the progression of Alzheimer's is among those not approved.
The SMC, the body which approves drugs for use in the health service, said there was "uncertainty" around the "modest clinical benefit" of the drug Lecanemab.
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has deemed the medication as efficient at slowing Alzheimer's disease.
The drug is the first Alzheimer's treatment of its kind to be licensed for use in Great Britain but it has not been rolled out in England or Wales either due to the cost.
Campaigners at Alzheimer Scotland said they were "disappointed" by the decision, which chief executive Henry Simmons said was "based on the medicine's cost in relation to the evidence of its clinical benefit".
He said: "We remain optimistic that these initial hurdles will be overcome and, after decades of waiting, that new treatments will be approved for NHS use soon."
Olaparib - which is also known under the brand name Lynparza - was approved for prescription to adults with breast cancer linked to the BRCA1 gene, or those with mutated HER2-negative advanced breast cancer.
Chief executive of Breast Cancer Now, Claire Rowney, said: "It's brilliant this targeted treatment has been made available on the NHS in Scotland.
"Crucially, it offers people living with incurable secondary breast cancer with an altered BRCA gene an additional drug option to help stop their cancer from progressing for longer, so they can continue doing the things that matter most to them."
In addition to olaparib, the SMC approved cemiplimab for treating women with recurrent or metastatic cervical cancer, where the cancer has progressed on or after chemotherapy.
It also backed the use of durvalumab as a treatment together with chemotherapy for those patients with newly diagnosed extensive-stage small cell lung cancer.
Meanwhile, fenfluramine was approved for use to help treat a serious, rare type of epilepsy called Lennox-Gastaut syndrome, with another drug, cabotegravir approved to help prevent sexually transmitted HIV infections in adults and adolescents who are at high risk of being infected.
Lastly, the SMC backed the use of netarsudil/latanoprost for patients suffering from high pressure in the eye or the eye condition glaucoma.
SMC chairman Dr Scott Muir said: "The committee was pleased to be able to accept six new medicines for use by NHS Scotland.
"Cabotegravir, when used together with safer sex practices may help to reduce the spread of HIV, which is an ongoing priority for the Scottish government.
"Cemiplimab offers a second line treatment option for patients with advanced cervical cancer, where there are few others."
